21-CFR Part 11 Data Loggers
We offer data loggers and monitoring systems from several different manufacturers that offer firmware/software to allow them to operate as part of an FDA 21 CFR Part 11 compliant system. They can be used in applications including pharmaceutical manufacturing and distribution, hospitals and life sciences, and food and beverage production.
Read More…
There are 3 important groups of features for compliance with the CFR requirements:
- User management with secure user login, multiple levels of access and automatic inactivity log out.
- Audit trail to provide a record of any changes to the configuration and settings of the system.
- Data storage in an unalterable forma to prevent after the fact modifications.
In addition to this there are several other features that provide additional functionality that most users find useful:
- Corrective action notes in the event of an alarm to enter information as to both the cause of the problem and what was done to resolve the issue.
- Built-in report generation for login activity, configuration changes, corrective action and monthly data summaries for record-keeping.
Read Less…
Showing all 16 results
-

Novus LogBox Wi-Fi
3 Universal Analog Inputs, WiFi and USB Interface. $388.00 -

Novus LogBox BLE
3 Universal Analog Inputs, Bluetooth and USB $353.00 -

Lascar EL-21CFR-2-LCD
21CFR compliant, LCD display, temp, humidity, dew point sensors, USB. $145.00 -

WiFi Bluetooth Dry Ice or Ultra-Low Freezer Monitoring Kit
Includes TR-75A, 1-2 Probes, Thermal Buffers, -80°C or Above Calibration $480.00 – $580.00 -

Brainchild PR20
5.6" Touch Screen, Paperless Chart Recorder, for Up to 24 Channel Inputs. $1,875.00 – $4,275.00 -

Novus LogBox LTE
2 Universal Analog Inputs, 1 Digital Input, 1 Digital Output, MQTT Protocol $388.00 -
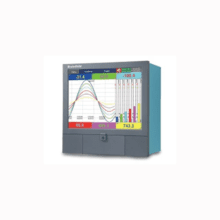
Brainchild PR30
12.1" Touch Screen, Paperless Chart Recorder, for Up to 48 Channel Inputs. $4,250.00 – $8,575.00 -

Delphin Expert Key 200
28 differential analog inputs, high accuracy 18 Bit A/D converter. -

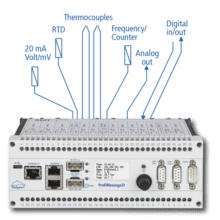
Delphin Expert Logger 400
Up to 16 analog input channels, 6 analog outputs, 1000Hz sampling rate. -

Delphin Expert Logger 300
Up to 46 analog input channels, 3000 m/sec sampling rate. -

Delphin Expert Logger 200
Up to 32 analog input channels, 2000 m/sec sampling rate. -

Expert Logger 100
Up to 16 analog input channels, 1000 m/sec sampling rate. -

Delphin Expert Key 100
14 differential analog inputs, high accuracy 18 Bit A/D converter. -

Brainchild PR10
4.3" Touch Screen, Paperless Chart Recorder, for Up to 6 Channel Inputs. $1,495.00 – $1,795.00