Identify and Help Prevent Risks in Hospital & Pharma Cleanrooms
 Many hospital and pharmaceutical technicians know that they need to establish an environmental monitoring plan/regime for their cleanroom, but CDC regulations are many and varied. How do you tie together the many different requirements to monitor, alarm and document the data?
Many hospital and pharmaceutical technicians know that they need to establish an environmental monitoring plan/regime for their cleanroom, but CDC regulations are many and varied. How do you tie together the many different requirements to monitor, alarm and document the data?
If you work in a cleanroom monitoring application such as a product fill cleanroom, in non-sterile manufacturing, or in a medical cleanroom, this latest White Paper from CAS DataLoggers can help you to get started on an actionable monitoring plan.
Environmental Monitoring Basics:
Whether you’re a Surgical Technician, Quality Engineer, or Pharmaceutical Microbiologist, passing your standards audit will require a comprehensive monitoring plan.
CDC (Centers for Disease Control and Prevention) guidelines on environmental measurement in cleanrooms and isolated areas state: “Do not conduct random, undirected, microbiologic sampling of air, water, and environmental surfaces in health-care facilities (270,343). Category IB.”
The main risk to cleanrooms is the loss or reduction of sterility assurance and the subsequent microbiological contamination that follows. Environments are monitored to protect pharmaceutical products from contaminants and to prevent OOS (out of specification), OOL (out of limit), and OOT (out of trend) alarm events from occurring. This is the equivalent of the modern healthcare industry placing its emphasis on prevention rather than cure.
Isolated areas require both air and surface monitoring to achieve sterilization/decontamination. Without recourse to historical data in the form of stored measurements, technicians lack any reliable way to determine sterilization effectiveness.
Carefully consider the requirements of your specific class of cleanroom as they relate to measurement collection. For example, when working in isolated environments graded Class A, C and D, personnel should routinely sample the air and swab the surfaces. If your class mandates the daily use of particle counters (Grade A esp.) then consider using an automated monitoring system with remote monitoring capability for instant access to alarms.
Note that settle plates, although often used as a simple way to roughly gauge the level of microbial contaminants, are not by themselves sufficient to ensure sterilization or to pass an audit. If you plan on using an environmental monitoring system, be sure to set it to monitor even throughout the duration of settle plate use. The CDC’s guidelines state: “Do not use settle plates to quantify the concentration of airborne fungal spores (348). Category II”
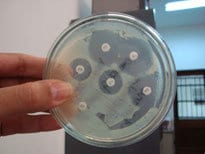 An especially high risk to cleanrooms is posed by gram-negative bacteria which contaminate water and produce most toxics found in the microclimate. It’s a common problem in aseptic processes, generating endotoxins which cause disease. Product fill cleanrooms in particular are a good breeding ground for these bacteria since there are often multiple exposed petri dishes located throughout the cleanroom for research purposes.
An especially high risk to cleanrooms is posed by gram-negative bacteria which contaminate water and produce most toxics found in the microclimate. It’s a common problem in aseptic processes, generating endotoxins which cause disease. Product fill cleanrooms in particular are a good breeding ground for these bacteria since there are often multiple exposed petri dishes located throughout the cleanroom for research purposes.
This and other risks can be addressed by developing your own incubation regime, which in many cases requires specialized equipment. For example the CDC recommends to: ”Use standard cleaning and disinfection protocols to control environmental contamination with antibiotic-resistant, gram-positive cocci (e.g., methicillin-resistant Staphylococcus aureus, vancomycin intermediate sensitive Staphylococcus aureus, or vancomycin-resistant Enterococcus [VRE]) (318,320–322). Category IB.”
Before you consider the further details of your monitoring plan, ask yourself, are you planning on taking measurements manually or automatically?
- Manually: A major source of contamination in labs is caused by personnel taking frequent manual measurements. The more frequent these manual samples are, the higher chance of microbial contamination.
- Automatically: Automated monitoring systems can provide your business or organization with the technology to cut labor costs, save time, and measure your critical values at high accuracy. Full regulatory compliance with patient privacy and electronic documentation standards is possible using all the advanced archival and documentation capabilities available with system software.
Monitor, Alarm and Validate
Regular environmental measurements, whether taken manually or performed by an automated monitoring system, form the foundation of your individual risk assessment strategy. When conducted at the appropriate times and frequencies, these measurements  give personnel time to take remedial action before product quality and/or environmental sterility are threatened.
give personnel time to take remedial action before product quality and/or environmental sterility are threatened.
For instant notification, many environmental monitoring systems feature wireless communication, giving users the ability to remotely receive alarms and monitor data in real-time. This capability is especially useful for maintaining Grade A cleanrooms and other areas with gram-negative bacteria.
Accurate data measurement is also useful for out of limit (OOL) and out of specification (OOS) investigations, which determine why a given alarm went off. Finally, monitoring systems save all data for documentation and audit/validation purposes.
Your environmental monitoring plan should contain these three key factors:
- Determine Your Monitoring Points:
Your facility’s monitoring point layout depends on the size and area of your isolated environment. First determine where you need to take readings or to install sensors in your given cleanroom, hospital corridor, surgery, or other area. Your layout also depends on the level of risk to patient health or to product quality, for example whether you have an open or a closed process. As a good guideline, you’re safer having more monitoring points rather than less.
Before placing sensors, it’s critical to determine the area’s risk level using a designation of Low, Mid or High. In high-risk cases such as gram-negative bacteria applications, your installed sensors need a high accuracy to address the main risk of losing sterility through microbiological contamination.
Prioritize these areas:
- Areas with higher travel/population (corridors, surgery prep areas, etc.)
- Areas with longer work or incubation durations
- Dirty or wet areas
- Any significant variations within rooms
Putting this type of forethought into sensor placement helps you to determine how best to detect specific fungi/bacteria in operation-dependent regimes. This includes not only surface measurements but also active air sampling (volumetric air-samplers, particle counters etc.).
To accommodate a wider sensor grid, cleanroom monitoring systems are available in configurations with anywhere from one to hundreds of inputs. This makes them ideal to monitor filling machine cleanrooms, ISO 5 product fill cleanrooms, test sample incubation, non-sterile manufacturing, and many other applications.
- Choose Monitoring Frequencies:
Also known as sampling rate, your monitoring frequencies should generally be performed frequently, at minimum recording a reading at each batch filling, at defined intervals, or immediately after sanitizing isolated & sterile areas.
You should also take a reading following:
- Essential maintenance
- Monthly/quarterly monitoring
- Incubation of test samples
- Critical times and temperature windows of operation-dependent regimes.
Monitoring systems can also accommodate different sampling rates for different cleanroom types. Your particular monitoring frequency will often depend on your individual cleanroom’s class, rated by the ISO (International Organization for Standardization). Example classes include:
- ISO 5: Common examples are applications with specified filling points in liquid containers. Monitoring frequency needs to be either daily or at the end of each batch, whichever is higher.
- ISO 7: This class includes gowning rooms and other applications which have surrounding areas rated at ISO5; here a weekly monitoring frequency is indicated.
Monitoring Systems and Sampling Rate:
As we covered in an earlier article, ‘How Fast Should You Sample on Your Thermocouples?,’ sampling rate is highly dependent upon your individual application needs.
Modern environmental monitoring systems can sample data once every second or faster, which is more than enough for most cleanroom applications. Note that a high sample rate is inversely tied to system battery life and the specifications of the specific device chosen. Recording duration can be determined by dividing the data logger’s memory capacity by its sample rate. However, many monitoring systems utilize a non-volatile memory to ensure that all recorded data is still safe if the battery fails or power is lost.
In most cleanroom applications, your temperatures won’t fluctuate several times a second. With this in mind, you can configure your data loggers at a lower sampling rate (say, once every five or ten minutes depending on the measurement value). This helps to save device memory space and time spent trending data during analysis.
Applications that may require faster sampling rates include advanced scientific research, high-speed equipment tests, etc. If you do need to sample at extreme rates of several readings a second, you’ll need to use small sensors, and the material you’re monitoring has to have a small thermal mass, unlike when monitoring typical industrial process temperatures.
- Documentation and Validation:
Full data documentation and subsequent validation is the third major component of an effective cleanroom monitoring regimen. For audit and standardization purposes, all your data and methodology needs to be recorded and validated, both for your own use and for inspectors. As part of this process, be sure to itemize your monitoring plan to give to auditors later.
Trending Environmental Data:
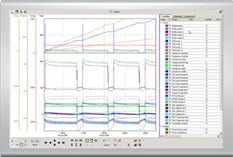 Armed with several months or a year’s worth of collected environmental data, you can, in many applications, examine it all in trend format to determine your plan’s effectiveness. Whether your regime is working as intended and you can consequently reduce sampling rate, or whether you find that your plan needs to be reconsidered, validation is a key reason to trend your data. Also be sure that your assembled data is easily accessible to inspectors on short notice.
Armed with several months or a year’s worth of collected environmental data, you can, in many applications, examine it all in trend format to determine your plan’s effectiveness. Whether your regime is working as intended and you can consequently reduce sampling rate, or whether you find that your plan needs to be reconsidered, validation is a key reason to trend your data. Also be sure that your assembled data is easily accessible to inspectors on short notice.
Any and all cleanroom validation solutions need to be able to automatically record, store, and trend your environmental data. By using data logger software, you can view historic data by specific filters. This is an easy way to view all alarm violations over a given period, which helps in OOS/OOL investigations to discover why alarms triggered in the first place.
As part of good manufacturing practice (GMP), be on the lookout for microflora and specifically for resistant bacterial strains. Unlike settle plates, trended data can give you a detailed view of microbial contamination levels, leading to final validation.
Summary:
Effective sterility assurance in cleanrooms and other isolated areas requires users to Monitor, Alarm and Validate as the three critical tenets of a comprehensive environmental monitoring plan. These mutually-complementary concerns give you actionable items to consider as you determine the ideal regime for your facility’s needs. With forethought and using a combination of sensors and software, you can help to ensure your facility’s environmental integrity and thereby achieve validation.
About the Authors:
CAS DataLoggers environmental monitoring systems are installed in a variety of hospital cleanrooms, pharmaceutical cleanrooms, government health centers, manufacturing plants, museums and galleries, and in all manner of additional applications.
To learn more about Cleanroom Monitoring Solutions, or to find the ideal solution for your application-specific needs, contact a CAS DataLoggers Application Specialist at (800) 956-4437 or request more information.

